COVID-19: Social Distancing & the Future of Pandemics
 Najung Lee (Pembroke) & Ragavi Vijayakumar (Downing). April 20, 2020.
Najung Lee (Pembroke) & Ragavi Vijayakumar (Downing). April 20, 2020.
Social distancing
Social distancing means keeping space between yourself and other people outside of your home. The basic reproductive number (R0) is the expected number of cases directly generated by one case. The Signer Laboratory has made the following assumptions1 in order to mathematically model the effects of social distancing on R0:- Under normal conditions, each infected person is expected to infect 2.5 other people.
- Infected people can transmit the disease for a five-day period while they are asymptomatic.
- After five days a person will begin experiencing symptoms, quarantine and no longer infect others.
- There is a direct linear correlation between social interaction and R0. For instance, R0 is reduced by 50% (R0=1.25) when social interactions are reduced by 50%.

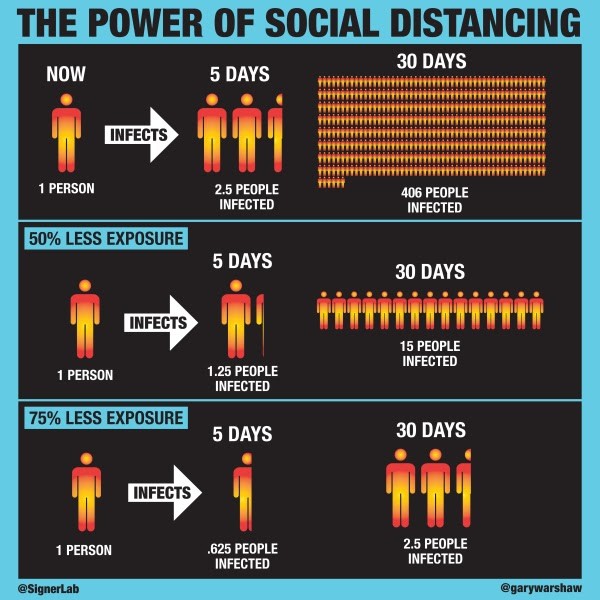
Figure 1. Simplified infographic on the impacts of social distancing1
The Future: Recurring global health threat and what do we need to learn
After the discovery of vaccines and antibiotics and with the improvement in hygiene, the number of deadly infectious diseases had rapidly declined. We were in hopes of eradicating them. Unfortunately, there have been new strains of infectious pathogens emerging from the 1970s and recently, the period between subsequent outbreaks has become shorter.Why is this phenomenon happening despite the remarkable development of medical technology? The common feature shared by most of the diseases is that they are zoonotic viruses, which means they can infect both animals and humans. Researchers found out that more than 60% of emerging infectious diseases (EIDs), whose incidence has increased in the past 20 years, are caused by zoonotic pathogens2.
HIV came from many cross-species transmission from primates in Africa3. H1N1 is a type of swine influenza virus (SIV), which is a strain of the influenza family of viruses that circulate in pigs4. SARS-CoV, MERS-CoV, and SARS-CoV-2 are originally bat-borne coronaviruses. The noticeable point here is that the viruses have all crossed the species barrier from their natural host, and this phenomenon of cross-species transmission is called ‘Spillover’.
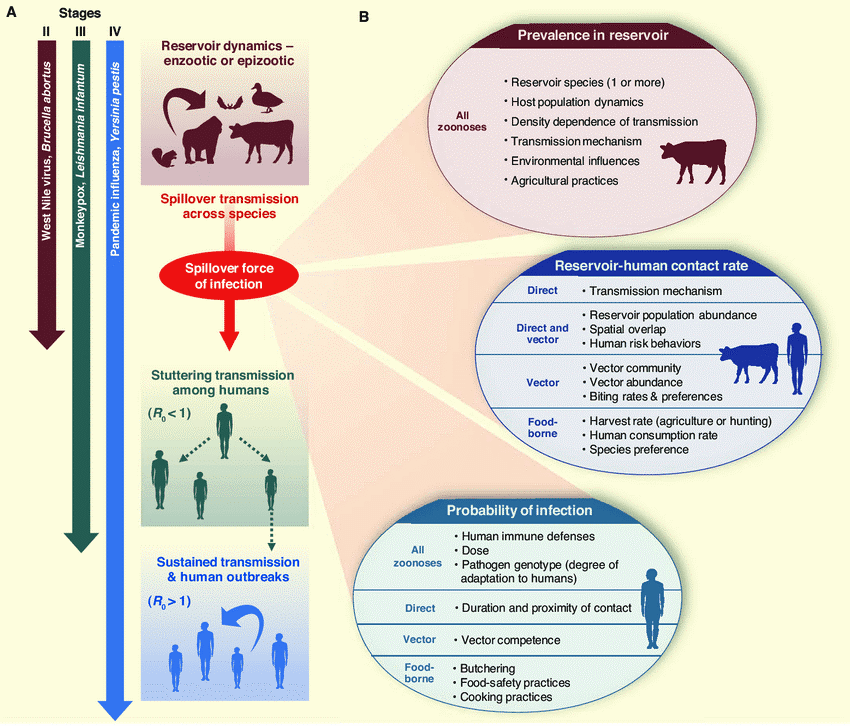
Figure 2.
Schematic diagram of zoonotic transmission dynamics12.
There exist other factors, primarily the increase in the frequency of human and wildlife contact, which is accelerating the emergence of novel outbreaks6. Deforestation, rapid urbanisation, bushmeat hunting, and wet markets are forcing wild animals to move away from their natural habitats and to have greater contact with humans. This increases the risk of Spillover as there is a greater chance for the mutated virus within wild animals, which could infect another animal species, to be transmitted.
Most of the zoonotic infection cases also involve ‘intermediate hosts’ that connect between natural hosts – which are reservoirs of different viruses – and humans. The intermediate hosts can amplify the pathogen transmission and/ or introduce a genetic variation7. The ‘mixer vessels’ species, such as pigs, can recombine different viruses and produce a completely new recombinant strain of virus that gives greater biological variation; the swine flu pandemic in 2009 was caused by a novel influenza virus that has obtained the ability to spread between humans by genetic reassortment of avian, human and/or swine flu viruses in pigs8. There are also many reported endemic cases with the likely source of human transmission being infected livestock9. This is maybe warning us that we are opening Pandora’s box ourselves by allowing pathogens to overcome the cross-species barrier and infect intermediate hosts, which can essentially be any animals around us.
Furthermore, a massive increase in the frequency of air travel is providing an optimum environment for rapid transmission of infectious disease not only within certain communities but also across the globe6. Therefore, it is worth noting that any contagious disease in a single region is not the problem of a specific country or area; rather, the entire world needs to collaborate to achieve ‘One Health,’ which is an objective by the World Health Organization (WHO) to achieve better public health outcomes10. In such a globalised world, a complacent attitude towards an outbreak might result in failure in early prevention. In this process, WHO should also need to take an active step in constructing a global network to identify the regions with potential risks and to circulate up-to-date information transparently and promptly.
The vast majority of the world has been focusing on post-outbreak responses to a pandemic such as the development of vaccines and medical treatments. However, pre-outbreak measures are also vital to prevent initial mass infection, which can easily lead to uncontrollable situations. Early detection, surveillance, and mass testing are essential to block the inflow and nation-wide spread of the disease by improving preventive measures against epidemics to minimise considerable damage.
What can we, as in individual, do during the period of the outbreak? Along with individual protection measures such as wearing masks and washing hands frequently, having correct information and knowledge about the infectious disease is indeed very crucial. Fake news with incendiary titles instigates the public, triggering fear and panic. Such behaviour rather hinders the effort made by scientists and the government to control the situation. Hence, greater engagement of the public to the scientific background of infectious disease would make us better prepared for the unknown future.
Disease X
In 2018, WHO announced “Disease X” – representing a hypothetical, unknown pathogen that could cause a serious international epidemic11. Currently, COVID-19 fits the Disease X category. There is no guarantee that a pandemic like COVID-19 would not happen again soon; “Disease X” can appear in any form at any time. We need to learn from what has happened and thoroughly prepare so that future outbreaks would not lead to disastrous consequences.The above article was written for the purposes of general public education about updates on the COVID-19 outbreak. It should not replace information provided by medical professionals and government officials.
References
1 Signer Laboratory. (n.d.).2,5 Jones, K. E., Patel, N. G., Levy, M. A., Storeygard, A., Balk, D., Gittleman, J. L., & Daszak, P. (2008). Global trends in emerging infectious diseases. Nature, 451(7181), 990–993.
3 Sharp, P. M., & Hahn, B. H. (2011). Origins of HIV and the AIDS Pandemic. Cold Spring Harbor Perspectives in Medicine, 1(1).
4 Smith, G. J. D., Vijaykrishna, D., Bahl, J., Lycett, S. J., Worobey, M., Pybus, O. G., … Rambaut, A. (2009). Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature, 459(7250), 1122–1125.
6Alirol, E., Getaz, L., Stoll, B., Chappuis, F., & Loutan, L. (2011). Urbanisation and infectious diseases in a globalised world. The Lancet Infectious Diseases, 11(2), 131–141.
7 Cui, J.-A., Chen, F., & Fan, S. (2017). Effect of Intermediate Hosts on Emerging Zoonoses. Vector-Borne and Zoonotic Diseases, 17(8), 599–609.
8 Kong, W., Wang, F., Dong, B., Ou, C., Meng, D., Liu, J., & Fan, Z.-C. (2015). Novel reassortant influenza viruses between pandemic (H1N1) 2009 and other influenza viruses pose a risk to public health. Microbial Pathogenesis, 89, 62–72.
9 Kilpatrick, A. M., & Randolph, S. E. (2012). Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. The Lancet, 380(9857), 1946–1955.
10 One Health. (n.d.).
11 List of Blueprint priority diseases. (2018, July 20).
12 Lloyd-Smith, J. O., George, D., Pepin, K. M., Pitzer, V. E., Pulliam, J. R. C., Dobson, A. P., … Grenfell, B. T. (2009). Epidemic Dynamics at the Human-Animal Interface. Science, 326(5958), 1362–1367.
Trackback from your site.
