COVID-19: Current and Potential Treatments

Ragavi Vijayakumar (Downing) & Najung Lee (Pembroke). April 13, 2020.
Current treatments
As of now, there is no vaccine against SARS-CoV-2 – the virus strain that causes COVID-19. Researchers are currently working on creating a vaccine specifically for this virus, as well as potential treatments for COVID-19. Antibiotics are ineffective because COVID-19 is a viral infection, not bacterial.There is some evidence that certain medications may have the potential to be effective in treating the symptoms of COVID-19. However, researchers need to perform properly randomized controlled trials in humans before these medications become available as a treatment method for COVID-191. It is also important to realise the fact that the efficacy and side effects of the drug can differ from person to person.
Here is a treatment option that is currently being investigated for protection against SARS-CoV-2 and treatment of COVID-19 symptoms.
Remdesivir
Remdesivir was initially developed by Gilead Sciences. It has a similar structure to adenosine, so it shuts down viral replication by inhibiting a key viral enzyme, RNA polymerase2,3. Researchers did testing with Remdesivir in the past during the Ebola outbreak, however, it did not show any promising improvement.
Remdesivir was given a chance to shine again. In the United States, a COVID-19 patient was given Remdesivir when his condition worsened; his symptoms showed improvements the next day, according to a case report in The New England Journal of Medicine (NEJM).
Such evidence from individual cases is not sufficient to prove a drug’s safety and efficacy, however. Still further testing on humans is required to make a safe conclusion before its global usage as a COVID-19 treatment.
APN01 – Apeiron Biologics
Apeiron is a privately-held European biotech company based in Vienna, Austria, focused on the discovery and development of novel cancer immunotherapies. It has recently secured approvals from regulatory agencies in Austria, Germany and Denmark to conduct a Phase II clinical trial of APN01 for the treatment of COVID-1915.
APN01 is a recombinant form of human angiotensin-converting enzyme 2 (ACE2). It was previously tested in phase I and II trials for acute lung injury (ALI) and pulmonary artery hypertension (PAH) involving 89 patients14.
The drug has been hypothesised to work against SARS-CoV-2 in two ways. Firstly, since it is a recombinant form of ACE 2, the virus binds to soluble APN01, instead of ACE2 on the cell surface, which means that the virus can no longer infect the cells.
At the same time, it reduces harmful inflammatory reactions in the lungs that occur in some patients with COVID-19 and lead to ALI and acute respiratory distress syndrome (ARDS).
Vaccines
What is a Vaccine?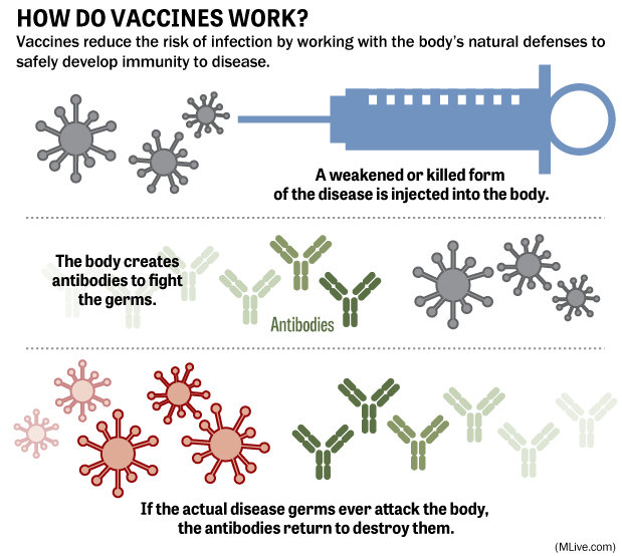
Figure 1. Simplified graphic on how vaccinations work4
A vaccine is typically a biological agent made from weakened or killed forms of the microbe, its toxins, or one of its surface proteins. It provides active acquired immunity to a particular infectious disease. It is important that the attenuation is done in a way that makes the pathogens incapable of causing an infection, but still able to induce an immune response to confer resistance4,5.
Vaccines encourage our adaptive immune systems to produce highly specific antibodies and immunological memory against potential future infection. Essentially, vaccines work by introducing protection without having to risk the initial exposure of the wild-type pathogen.
How is a Vaccine produced?
The Centre for Disease Control has stated that there are six stages to vaccine development: Exploratory, Pre-Clinical, Clinical Development, Regulatory Review and Approval, Manufacturing and Quality Control. On average, developing and manufacturing a vaccine takes about 8 to 12 years in total.
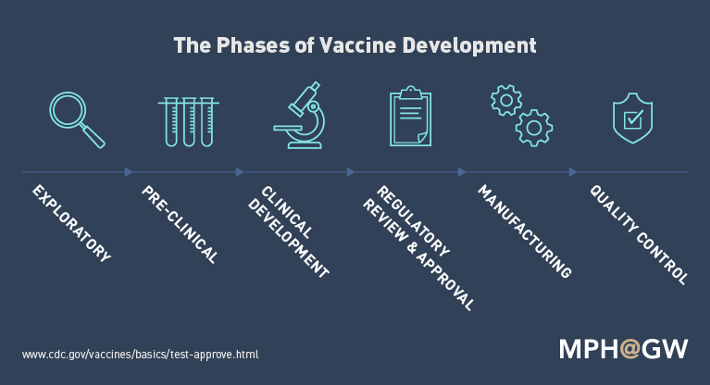
Figure 2. Simplified graphic on the phases of vaccine development6
Exploratory: This phase is to characterize the pathogen and identify potential antigens that might help treat or prevent a disease6.
Pre-clinical: This phase is to determine if the potential antigen has the ability to produce immunity, while not causing harm through animal testing.
Clinical development: An application for an Investigational New Drug (IND) to the U.S. Food and Drug Administration (FDA) is made. This application basically summarizes all the pre-clinical findings to date and also describes how the drug will be tested and created.
Once the proposal has been approved, the vaccine must pass three trial stages of human testing:
- Phase I: administers the candidate vaccine to a small group (less than 100 people) with the goal of determining whether the candidate vaccine is safe and to learn more about the responses it provokes among test subjects.
- Phase II: which includes hundreds of human test subjects, aims to deliver more information about safety, immunogenicity, immunization schedule and dose size.
- Phase III: which can include thousands or tens of thousands of test subjects, continues to measure the safety (rare side effects sometimes don’t appear in smaller groups) and effectiveness of the candidate vaccine.
Manufacturing: Drug manufacturers provide the necessary support to create mass quantities of vaccines.
Quality control: Stakeholders – healthcare system and providers, academic researchers, vaccine manufacturers, etc. – involved must adhere to procedures that allow them to monitor whether the vaccine is performing as well as anticipated. Multiple systems are designed to keep track of its performance, safety and effectiveness of an approved vaccine7.
mRNA Vaccines
What is an mRNA vaccine?Instead of standard vaccines where viral proteins are used to immunize, an mRNA vaccine provides a synthetic viral mRNA, which the host body uses to produce viral proteins. This will allow the host body to produce necessary antibodies and immunological memory against a potential future infection8.
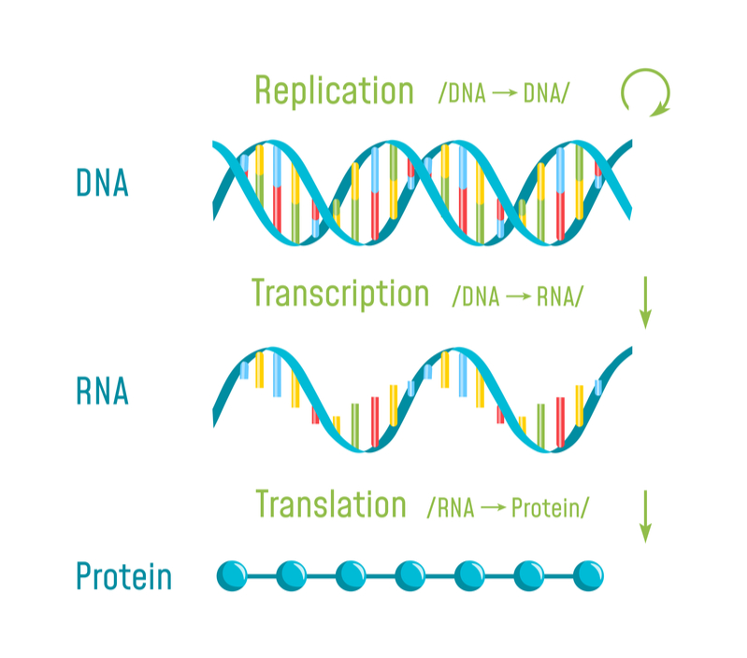
Figure 3. Diagram of Central Dogma
Smith, J. (2020, April 1). Analysis: Could mRNA Vaccines Fulfill Their Potential Against Coronavirus? Retrieved from https://www.labiotech.eu/medical/coronavirus-mrna-curevac-etherna/.
Advantages of mRNA vaccine
mRNA vaccines:
- are much safer than killed or attenuated viruses since it is non-infectious and non-integrating. There is close to no risk of infection or insertional mutagenesis
- can be administered repeatedly
- have the potential for rapid, inexpensive and scalable manufacturing, mainly owing to the high yields of in vitro transcription reactions. It bypasses the process of producing and purifying viral proteins for vaccines, saving time for production.
mRNA molecules are highly unstable since they are susceptible to degradation in the cytoplasm after a short period of time. They also have high innate immunogenicity which needs to be downregulated for the safety of the vaccine. Finally, in vivo delivery of mRNA molecules is inefficient making it a bad candidate for vaccines9.
How can these problems be overcome?
mRNA degradation can be regulated using various chemical modifications:
- addition of 5’ CAP,
- adding an optimal length of poly(A) tail,
- replacing rare codons with frequently used synonymous codons that have abundant cognate tRNA in the cytosol.
Immunogenicity of the mRNA can be down-modulated to further increase the safety profile.
The efficiency of in vivo delivery can be increased through the insertion of mRNA into carrier molecules – liquid nanoparticles, so that mRNA is in an injectable form – will allow rapid uptake and expression in the cytoplasm.
Moderna, Inc. – Developing an mRNA vaccine (mRNA-1273)
Moderna, Inc. is a Cambridge, Massachusetts-based biotechnology company that is focused on drug discovery and drug development based on messenger RNA. Moderna is in the process of developing an mRNA vaccine, mRNA-1273, which encodes for the SARS-CoV-2 spike protein. The first participant of the mRNA-1273 was dosed on the 16th March 2020 (Phase I trials). There’s still Phase II and III to overcome, but if every stage of the vaccine development goes smoothly, mass production of the SARS-CoV-2 vaccine could start in about a year or a year and a half, at the earliest10,11.
Another concept? RNA-based Antibodies
Moderna Inc. is also working on mRNA vaccines which encodes an antibody protein known to attack the virus. Effective antibodies can be identified from those who have gotten immunity through infection and recovery of COVID-19. Specific antibodies against SARS-CoV-2 can be isolated and can be sequenced, such that the mRNA sequences for the antibody is identified. These mRNA, if injected into an individual, will be translated into antibody against proteins on the virus itself, conferring immunity to the disease12.
CanSino Biologics, Inc. – Developing a Viral Vector-Based Vaccine (Ad5-nCoV)
CanSino Biologics Inc. is a global vaccine company based in China. CanSino Biologics is in the process of developing a recombinant SARS-CoV-2 vaccine (adenovirus type 5 vector) candidate. In preclinical animal studies of Ad5-nCoV, the vaccine candidate was able to trigger a strong immune response and a satisfactory safety profile. It has recently received Chinese regulatory approval to start human trials13.
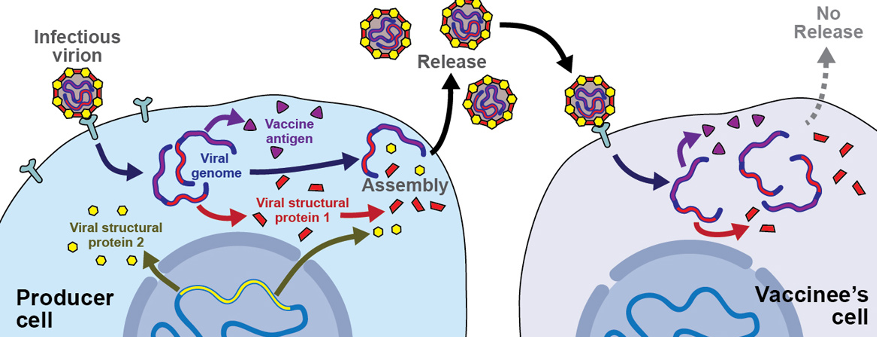
Figure 4. Diagram of Vector-Based Vaccine
D., D. (2017, August 8). Virally vectored vaccine delivery: medical needs, mechanisms, advantages and challenges. Retrieved from https://smw.ch/article/doi/smw.2017.14465.
This article has touched on some of the major discoveries by some companies/research groups around the world to prevent and fight COVID-19. It is just an overview, however, and the actual progress is far beyond what this article is able to cover.
The above article was written for the purposes of general public education about updates on the COVID-19 outbreak. It should not replace information provided by medical professionals and government officials.
References
1Yetman, D. (2020, April 6). Coronavirus Treatment: How Is COVID-19 Treated?2Kupferschmidt, K., CohenMar, J., BrainardApr, J., HeidtApr, A., Ortega, R. P., CleryApr, D., & Ortega, R. P. (2020, March 27). WHO launches global megatrial of the four most promising coronavirus treatments.
3Wang, M., Cao, R., Zhang, L. et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30, 269–271 (2020).
4Klingensmith, M. (2014, December 10). How do vaccinations work? The science of immunizations.
5Federman RS. Understanding vaccines: a public imperative. Yale J Biol Med. 2014;87(4):417-22.
6Producing Prevention: The Complex Development of Vaccines. (2019, March 6).
7How we develop new vaccines. (n.d.).
8Belluz, J., Irfan, U., & Resnick, B. (2020, March 27). A guide to the vaccines and drugs that could fight coronavirus.
9Pardi, N., Hogan, M. J., Porter, F. W., & Weissman, D. (2018, April). mRNA vaccines – a new era in vaccinology.
10LeMieux, J. (2020, March 26). Moderna’s SARS-CoV-2 Vaccine’s Fast Track to Clinical Trials.
11BioSpace. (2020, March 16). Moderna Announces First Participant Dosed in NIH-led Phase 1 Study of mRNA Vaccine (mRNA-1273) Against Novel Coronavirus.
12Mishra, S., Carnahan, R., & Postdoctoral Scholar of Pathology. (2020, April 10). Coronavirus: A new type of vaccine using RNA could help defeat COVID-19.
13(n.d.).
14Taylor, P. (2020, April 5). Apeiron starts mid-stage trial of drug that blocks coronavirus.
15Technology Networks. (2020, April 2). Phase 2 Clinical Trial of APN01 for Treatment of COVID-19 Inititated.
